Impact of Spacer Design on Respiratory Drug Delivery and Potential Drug Cost Implications
Nagel M1 , Sartori S1 , Rozanski EA2
American College of Veterinary Internal Medicine (ACVIM). 2020.
1. Trudell Medical International 2. Cummings School of Veterinary Medicine at Tufts
INTRODUCTION
- Valved holding chambers, or ‘spacers’, enable inhaled medications to be delivered to animals.
- Inhaled steroids are effective therapeutics for the management of respiratory diseases and are an alternative to systemic steroids which may have short and long term side effects.
- However, the delivery process is a critical component of aerosol drug delivery and must be considered when assessing drug performance.
- Chambers are designed to decelerate and capture the aerosol plume from a Metered Dose Inhaler(MDI) in order to alleviate coordination issues to give animals time to inhale the medication properly.
OBJECTIVE
- Inhaler delivery performance, which impacts pulmonary deposition and desired therapeutic effect, can be influenced by inhalation chamber design elements such as volume and length, the use of electrostatic dissipative materials, inhalation valve function, and the design of the mask interface.
- The purpose of this in vitro study was to assess drug availability in a range of chambers in a clinically relevant setting to provide guidance to veterinarians when recommending a chamber.
METHODS
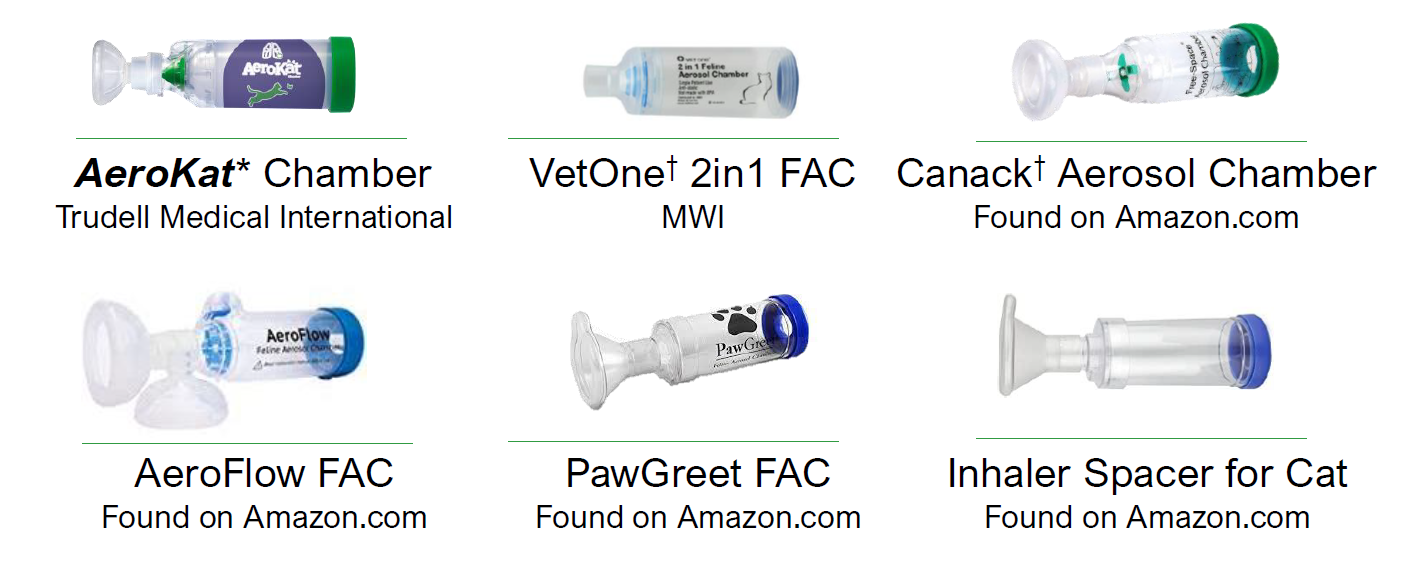
- This study evaluated the impact of drug availability when using fluticasone ( Flovent HFA 110 μ g) MDI with a range of different chambers available on the market.
- Each chamber was evaluated by breathing simulator programmed to simulate the tidal breathing pattern of a cat (tidal volume 50 mL, Inspiratory/expiratory ratio = 1:2, 20 breaths per minute).
- Coordination delays between MDI actuation and inhalation by tidal breathing were imposed to simulate real-world use conditions.
- Delays of 2, 5, and 10 seconds were investigated to determine the impact on delivered drug mass.
- The breathing simulator was coupled to a filter which in turn was attached to the mouthpiece of the device on test.
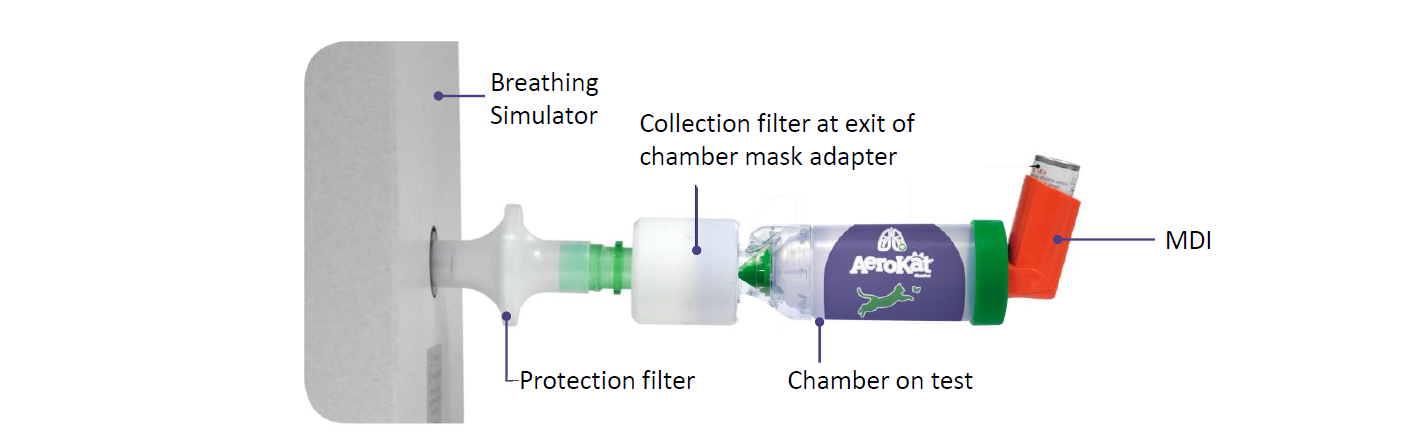
- The mass of drug collected on the filter is indicative of total drug availability to the animal.
- Following activation of the MDI and appropriate delay interval, the filter was subsequently assayed via high performance liquid chromatography and the mass of fluticasone propionate determined.
RESULTS
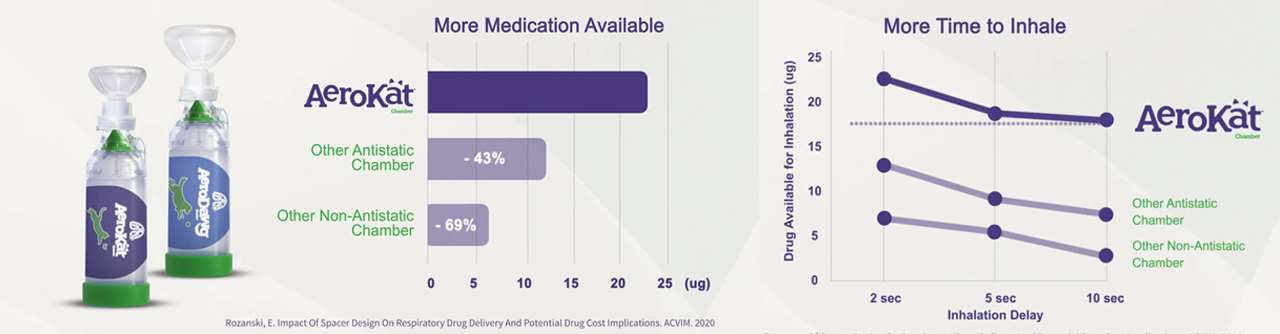
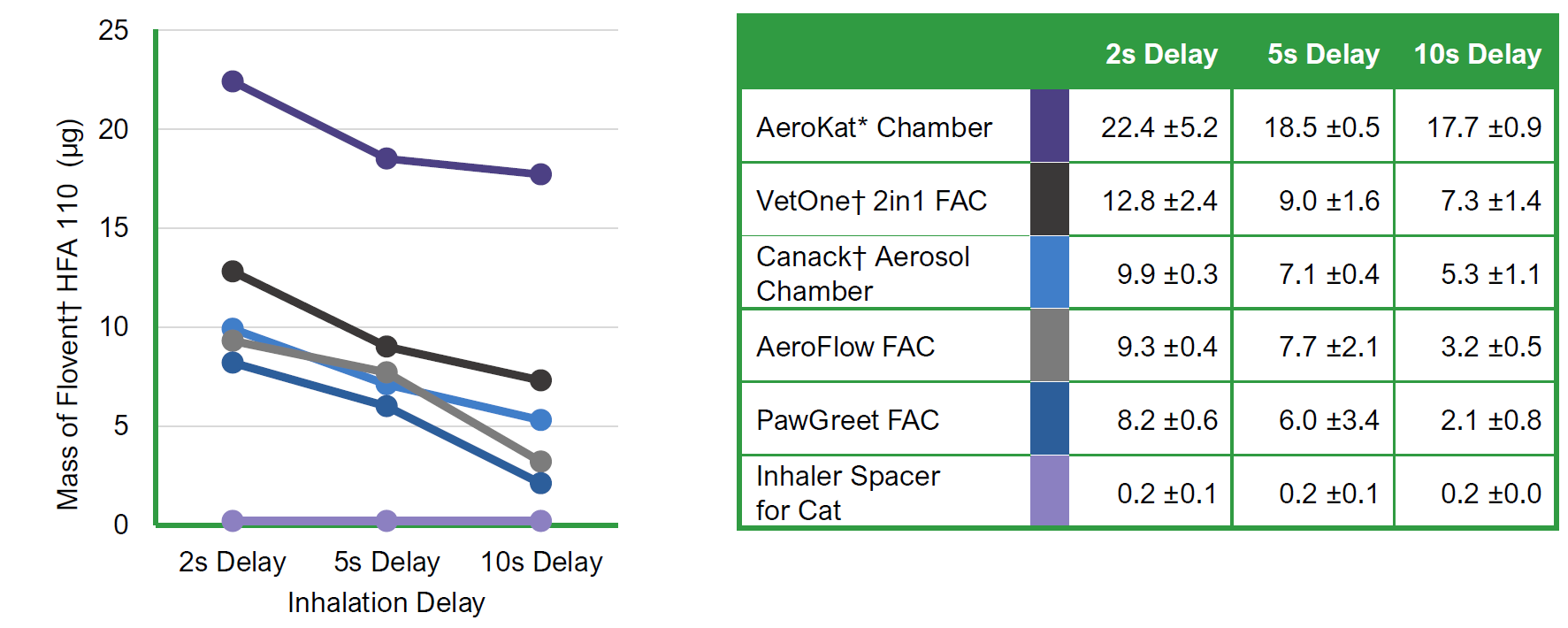
Drug Availability Over Time from Inhalation Chambers
DISCUSSION
- Different chambers deliver different amounts of medication.
- Chambers that make more medication available for a longer time may better support how animals’ interface with these devices. For example:
- the inhaler may be activated before the mask is applied;
- animals with small tidal volumes take longer to empty the chamber; or,
- animals may briefly hold their breath. Availability of medication has implications for disease control and owner expense.
- Animals not well controlled may experience more symptoms or need higher doses of medication to control the animal’s disease. For example:
- using the 2s Delay results and AeroKat* Chamber as a reference, owners delivering a Flovent† HFA 110 inhaler (2 puffs per day, 60/month, $280 per MDI) could experience increased drug costs of between $1,246 -$3,774 annually.
CONCLUSION
- With new products entering the veterinary sphere and increasing access to online selling marketplaces, chambers need to be evaluated in order to validate drug delivery performance.
- Without this information, chambers that appear similar but perform differently could mean that some patients will not receive the intended dose and as a result may need their dose modified or be at heightened risk of poor disease control and potential for respiratory exacerbation.
